Most people assume that if a drug is approved by the FDA or EMA, it’s been thoroughly tested for every possible risk. But the truth is, some of the most dangerous drug interactions aren’t found until after millions of people start taking the medication. These are called post-market drug interactions - and they’re more common than you think.
Why Clinical Trials Miss Dangerous Interactions
Clinical trials are designed to prove a drug works and is generally safe. But they’re not built to catch everything. Most trials involve between 1,000 and 5,000 people, often healthy adults with one primary condition. They last six to twelve months. That’s not enough time or diversity to spot problems that only show up in older patients, people with multiple chronic diseases, or those taking five or more medications at once. For example, the drug benfluorex (sold as Mediator) was used by over 5 million people in Europe over 30 years before it was pulled in 2009. Why? Because it caused severe heart valve damage - a side effect that never showed up in trials. The same thing happened with pergolide, a Parkinson’s drug withdrawn in 2007 after it was linked to heart valve problems in about 1 million patient-years of use. Even common drugs like simvastatin (Zocor) and atorvastatin (Lipitor) had hidden dangers. Before post-market reports, no one knew that antifungal meds like fluconazole or even grapefruit juice could cause statin levels to spike by 3 to 15 times. That leads to rhabdomyolysis - a life-threatening muscle breakdown that can destroy your kidneys.How Post-Market Surveillance Works
After a drug hits the market, systems like the FDA’s FAERS (FDA Adverse Event Reporting System) and the EU’s EudraVigilance start collecting reports from doctors, pharmacists, and patients. These aren’t just complaints - they’re signals. When enough people report the same problem - say, muscle pain after taking simvastatin with an antifungal - regulators dig deeper. The FDA’s Sentinel Initiative monitors over 300 million patient records across hospitals and insurers. It uses real-world data to spot patterns no trial ever could. In one case, 2,847 reports of rhabdomyolysis linked to statin interactions were flagged between 2015 and 2020. Simvastatin with azole antifungals made up 37% of those cases. That’s how we learned to warn patients: Don’t take these together. AI is now helping too. Since 2023, the FDA has approved AI platforms that can process 10,000 adverse event reports a day with over 92% accuracy. In pilot studies, these tools cut signal detection time from 18 months to just 45 days. That’s a game-changer.Three Types of Post-Market Interactions
Not all interactions are the same. They fall into three categories:- Drug-drug interactions: One medication changes how another works. Like fluconazole blocking the liver enzyme CYP3A4, which normally breaks down simvastatin. Result? Toxic buildup.
- Drug-condition interactions: Your health condition makes a drug riskier. For example, someone with kidney disease taking apixaban (Eliquis) might bleed more easily - a risk not fully seen in trials with healthy kidneys.
- Drug-food interactions: Grapefruit juice is the classic example. It blocks CYP3A4 enzymes, so atorvastatin levels can jump 15-fold. Even one glass can be dangerous. Other culprits include alcohol (which can cause "dose dumping" with extended-release painkillers like Exalgo) and St. John’s Wort (which can make blood thinners like apixaban useless - or worse, trigger bleeding).
Real Stories Behind the Numbers
Behind every statistic is a person. Reddit threads are full of them. One user, u/MedSafety456, wrote in November 2022: "My doctor didn’t warn me about the grapefruit interaction with my Lipitor - ended up in the ER with kidney damage from rhabdomyolysis." Another case documented by the FDA involved a 78-year-old who started apixaban while still taking St. John’s Wort. The interaction wasn’t clearly labeled. He suffered life-threatening bleeding. On the flip side, tools like GoodRx’s interaction checker have saved lives. One user wrote on Trustpilot: "The interaction warning prevented me from taking ciprofloxacin with my blood pressure meds - my pharmacist confirmed it could have caused dangerous QT prolongation." That’s the power of accessible, real-time data.What’s Being Done to Fix the Gaps
The system isn’t perfect - but it’s getting better. Since 2021, the FDA requires post-approval studies for nearly half of all new drugs. Over 22% of those specifically require enhanced monitoring for drug interactions. The EU mandates risk management plans for every approved drug. Japan followed suit in 2012. Pharmaceutical companies are investing heavily. The global pharmacovigilance market jumped from $5.8 billion in 2020 to $7.3 billion in 2022. Big players like Parexel now make over $1 billion a year from safety monitoring services. Smaller biotechs outsource this work - because the cost of missing a dangerous interaction can be billions in lawsuits and recalls. New tech is helping too. The FDA’s Drug Interaction API handles 2.5 million queries daily from electronic health records. It warns doctors in real time when a prescription might clash with another drug. Blockchain systems, planned for rollout by 2025, aim to fix underreporting - currently, 90-95% of adverse events go unreported.
What You Can Do
You don’t have to wait for regulators to catch up. Here’s how to protect yourself:- Always tell your doctor and pharmacist every medication you take - including supplements, OTC painkillers, and herbal products like St. John’s Wort.
- Ask: "Could this interact with anything else I’m taking?" Don’t assume they know. Many doctors don’t have time to check every interaction.
- Use a reliable interaction checker - GoodRx, Medscape, or your pharmacy’s app. These tools flag risks before you fill the prescription.
- Know your red flags: Unexplained muscle pain, dark urine, dizziness, unusual bleeding, or sudden fatigue could signal a dangerous interaction.
- Don’t ignore food warnings. Grapefruit juice isn’t just a "maybe." It’s a hard no for many statins, blood pressure meds, and anti-anxiety drugs.
The Bigger Picture
Post-market drug interactions aren’t a flaw in the system - they’re a reminder that medicine is living science. Drugs behave differently in the real world than in a controlled trial. That’s why surveillance isn’t optional. It’s essential. The cost of getting this wrong is high: the Institute of Medicine estimated that drug interactions cost the U.S. healthcare system over $1 billion a year. And they’re mostly preventable. The future? Genomics. The NIH’s Pharmacogenomics Research Network is already studying how your genes affect how you metabolize drugs. In five years, your pharmacist might know not just what you’re taking - but how your DNA will react to it. For now, the best defense is awareness. Stay informed. Ask questions. Use the tools available. Because when it comes to your meds, the real test doesn’t begin until after the approval stamp - and that’s when you need to be your own best advocate.How common are drug interactions discovered after a drug is on the market?
About one-third of new drugs approved over the past decade had a major safety event after launch - like a black box warning, recall, or safety alert. Nearly 20% of new medications received a black box warning only after being used by millions of patients. Drug interactions are a leading cause of these events.
Why don’t drug companies find these interactions before approval?
Clinical trials are too small, too short, and too homogenous. They rarely include elderly patients, people with multiple chronic conditions, or those taking five or more drugs. Interactions that only show up after years of use - or in specific genetic groups - simply don’t appear in trials. That’s why real-world data is critical.
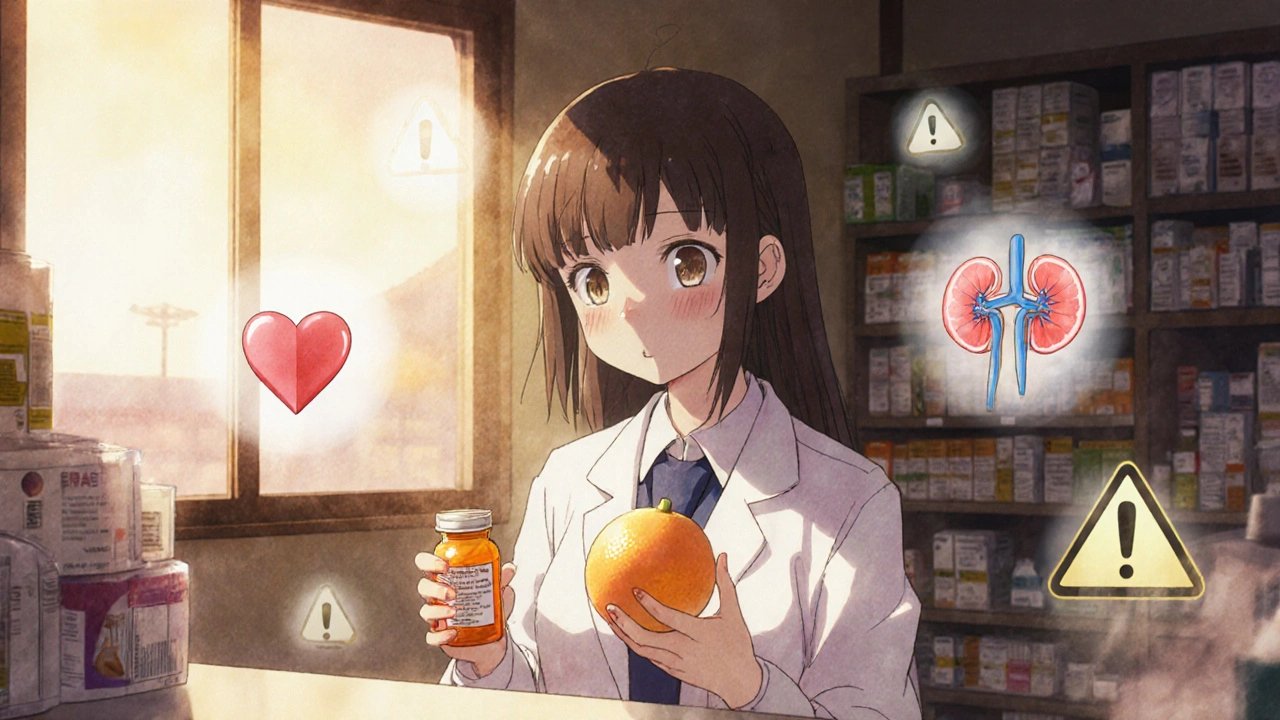
Can grapefruit juice really be dangerous with my medication?
Yes - and it’s not just grapefruit. Seville oranges, pomelos, and some grapefruit juices block the CYP3A4 enzyme in your liver, which breaks down dozens of medications. For statins like Lipitor or Zocor, this can raise drug levels by up to 15 times, leading to muscle damage or kidney failure. Even one glass can be risky. Always check if your drug has a grapefruit warning.
What should I do if I think I’m having a drug interaction?
Stop taking the medication immediately and contact your doctor or pharmacist. Don’t wait. Symptoms like unexplained muscle pain, dark urine, dizziness, unusual bleeding, or sudden fatigue could be signs of a serious reaction. Report the event to the FDA’s MedWatch program - your report helps protect others.
Are herbal supplements safe to take with prescription drugs?
Not always. St. John’s Wort can make blood thinners like apixaban or warfarin useless - increasing stroke risk. It can also interfere with antidepressants, birth control, and transplant drugs. Garlic, ginkgo, and ginger can increase bleeding risk when taken with anticoagulants. Always disclose every supplement you take - they’re not harmless just because they’re "natural."






9 Comments
They’re hiding the truth… again. The FDA? A puppet of Big Pharma. They knew about the grapefruit-statin link for years-suppressed it. Why? Because profits > lives. And don’t get me started on AI ‘solutions’-algorithms trained on corrupted data, fed by corporate lobbyists… It’s all a performance. You think your pharmacist’s app is helping? It’s a trap. They want you dependent. Watch your back. Always. Always. Always.
OMG I JUST REALIZED I’VE BEEN DRINKING GRAPEFRUIT JUICE WITH MY LIPITOR FOR 3 YEARS 😱😭 My pharmacist never said a word… I’m gonna scream. But also-thank you for this post. Someone finally said it out loud. 💪❤️
The systemic failure in pharmacovigilance is not accidental-it is structural. Clinical trials are designed for regulatory compliance, not human complexity. The fact that we rely on post-market surveillance as a safety net reveals a fundamental epistemological flaw in modern medicine: we treat drugs as discrete variables, not as elements within a dynamic biological ecosystem. This is not negligence-it is ideology. We must demand longitudinal, diverse, real-world data collection as a precondition for approval, not an afterthought. The cost of inaction is measured in kidneys, muscles, and lives.
Why do we even trust these f***ing agencies? They let 5 million people take Mediator for 30 years? That’s not incompetence-that’s treason. And now they wanna use AI to fix it? LOL. We need to burn the whole system down and start over. USA is the only country that still pretends this works. Rest of the world knows better.
CYP3A4 inhibition is the primary mechanism. Statins are hepatically metabolized. Fluconazole is a potent inhibitor. Pharmacokinetic synergy = toxic accumulation. Rhabdomyolysis risk escalates exponentially. Clinical trials lack polypharmacy cohorts. Ergo, post-marketing surveillance is non-negotiable. QED.
Who cares? Pharma is a scam. All drugs are poison. You think your ‘safe’ meds aren’t killing you slowly? Wake up. The FDA is owned by Pfizer. The EU? Merck’s lapdog. Even your ‘natural’ supplements? Designed to make you dependent. This post is just another distraction. The real problem? You’re all too dumb to realize you’re being farmed.
Let me be brutally honest-you people are sheep. You trust doctors? You trust ‘science’? You think a 12-month trial with 5,000 healthy 30-year-olds represents the 78-year-old diabetic with 7 prescriptions? You’re not just naive-you’re dangerous. The system is designed to fail you. They don’t want you healthy-they want you compliant. And now you’re reading about grapefruit juice like it’s a lifestyle tip? Pathetic. The real solution? Stop taking everything. Go raw. Fast. Meditate. Your body doesn’t need chemicals-it needs consciousness. But you? You’ll keep swallowing pills until your kidneys give out. And then? You’ll blame the FDA.
It’s a paradox of modern medicine: the more we know, the more we realize how little we understand. We quantify, we model, we algorithmize-but the human body remains an orchestra of variables we can’t fully score. Perhaps the true innovation isn’t in AI or blockchain, but in humility. To admit that safety is not a checkbox, but a continuous conversation between patient, practitioner, and the unknown.
Always tell your doctor and pharmacist everything you're taking-even the herbal stuff. It saves lives.
Write a comment