When you buy a bottle of medicine, a car part, or even a smartphone, you expect it to work exactly as it should. That’s not luck. It’s quality control testing-a quiet, systematic effort happening behind the scenes in factories around the world. In generic manufacturing, where cost efficiency and consistency are critical, skipping QC steps isn’t just risky-it’s financially dangerous. A single defective batch can cost hundreds of thousands in recalls, regulatory fines, or lost trust. The good news? With the right steps, you can catch problems early, cut waste, and build products people rely on.
Define Clear Quality Standards
Before a single part is made, you need to know what “good” looks like. This isn’t vague. It’s measurable. For example, in pharmaceutical manufacturing, a tablet’s weight must be within ±5% of its target. In electronics, a solder joint’s height must be between 0.3mm and 0.7mm. These numbers come from engineering specs, regulatory rules (like FDA 21 CFR Part 211), or industry standards like IPC-A-610 for electronics or ISO 9001:2015 for general manufacturing.Don’t assume everyone knows the standard. Write it down. Include tolerances, acceptable surface finishes (like Ra values under 3.2 μm), color consistency (ΔE < 2.0 on the CIELAB scale), and even how to handle samples. If you’re making a plastic housing, specify gloss levels in GU units. If it’s a metal component, define tensile strength targets with a ±5% margin. Without these, inspectors are guessing-and guessing leads to errors.
Implement the Right Inspection Methods
Once standards are set, you choose how to check them. Not every product needs 100% inspection. It depends on risk. For a pacemaker lead? Every single unit gets checked. For a plastic bottle cap? Random sampling using ANSI/ASQ Z1.4-2013 is standard.Tools vary by industry. In automotive, laser scanners measure dimensional accuracy down to ±0.005mm. In pharma, spectrometers verify chemical composition per ASTM E415. For visual defects, trained inspectors use magnifiers and light boxes following IPC-A-610 guidelines. Automated optical inspection (AOI) systems now scan circuit boards in seconds, spotting missing components or misaligned solder.
Some tests are physical: pulling a wire to check tensile strength, dropping a device to test durability, or heating a seal to confirm it won’t fail under stress. Others are electronic: measuring resistance, capacitance, or signal integrity. The key is matching the tool to the risk. If a defect could cause injury or regulatory failure, don’t cut corners.
Train Your Team Thoroughly
No matter how good your tools or standards are, if the person doing the test doesn’t know how to use them, it’s useless. Training isn’t a one-hour PowerPoint. It’s hands-on, repeated, and certified.At a typical electronics factory, inspectors get 24-40 hours of training before they touch a product. They learn how to calibrate micrometers, how to interpret AQL sampling tables, and how to document findings in digital logbooks. In pharma, operators must pass competency assessments tied to 21 CFR Part 11 compliance for electronic records.
Best practice? Pair new hires with experienced QC technicians for two weeks. Track their inspection accuracy over 50 samples. If they miss more than 2 defects, retrain. Top manufacturers aim for 95%+ certification rates. And don’t forget refreshers. Every six months, run a “defect challenge” where teams try to find hidden flaws in sample units. It keeps skills sharp.
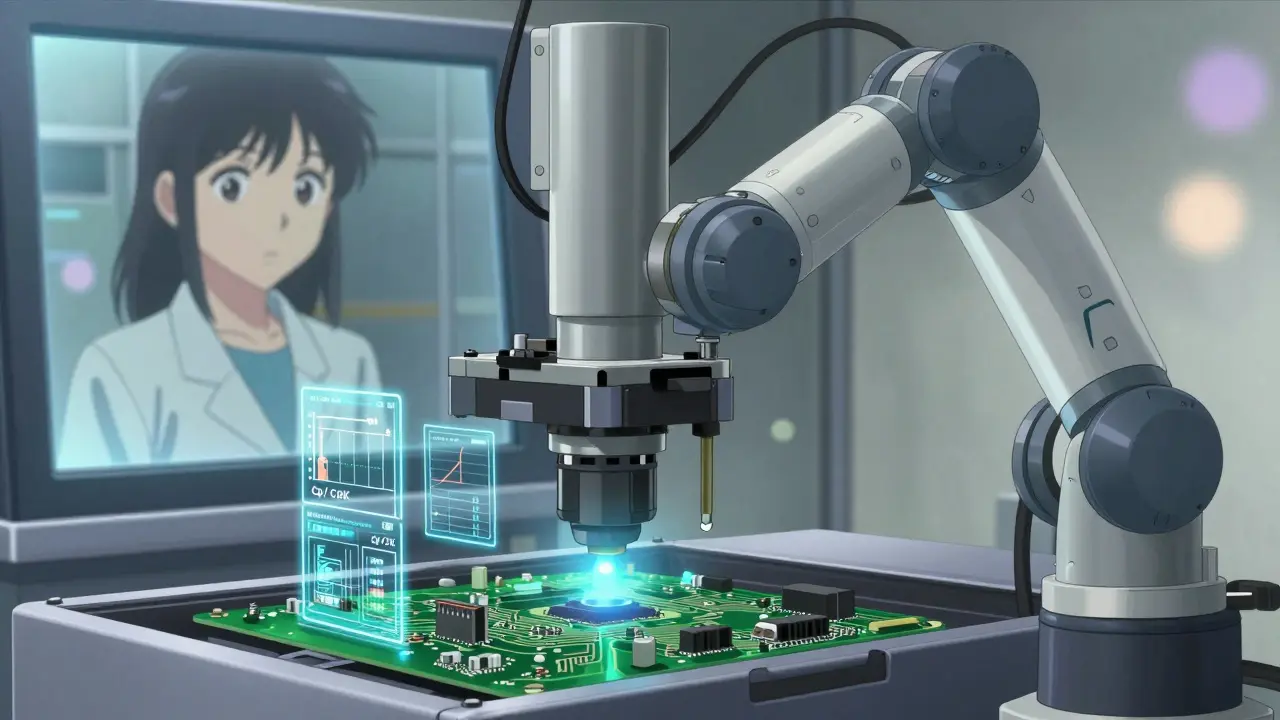
Monitor Processes in Real Time
Waiting until the end of the line to find defects is like checking your car’s brakes after it’s already skidding. Modern QC uses real-time monitoring at critical control points.On a production line, sensors track temperature, pressure, vibration, and speed. If a machine starts drifting-say, a molding machine’s cycle time increases by 0.5 seconds-a system flags it before the first bad part is made. Statistical Process Control (SPC) charts, like X-bar and R charts, plot data over time. If points go beyond the 3σ control limits, the process is out of control. That’s not a suggestion to stop-it’s an alarm.
Companies like Siemens use IoT sensors on every machine in their Amberg plant. Data flows into dashboards showing Cp/Cpk values in real time. A Cp/Cpk above 1.33 means the process is capable. Below that? You’re making too many defects. That’s why 65% of manufacturers plan to use IoT data for QC by 2026, up from just 28% in 2022.
Analyze Results with Data, Not Guesswork
Collecting data is easy. Using it wisely is hard. Many factories still use spreadsheets. That’s outdated. Use software like Minitab or JMP to find patterns.For example, if 70% of defects occur on Mondays, maybe shift changeovers aren’t being cleaned properly. If surface scratches cluster around Station 3, maybe a clamp is misaligned. Root cause analysis isn’t about blaming people-it’s about fixing systems. The FDA saw 43% of its 2021 Form 483 observations were due to poor test method validation. That means the tools themselves weren’t reliable. Always validate your test methods before using them.
Also, track defect types. Are you getting more dimensional errors? More contamination? More labeling mistakes? Each points to a different fix. A 2022 ASQ report showed companies using data-driven QC reduced scrap and rework by 32.7% on average. That’s not magic-it’s math.

Take Corrective Action-Fast
Finding a problem is only half the job. Fixing it before it repeats is the other half. That’s CAPA: Corrective and Preventive Action.When a defect is found, you don’t just throw out the batch. You ask: Why did this happen? Was it the material? The machine? The operator? The environment? You investigate. You fix the root cause. You update the procedure. And you verify the fix works.
Pharmaceutical companies must complete investigations within 72 hours. Other industries follow similar timelines. Delayed fixes mean more bad products, more customer complaints, and more regulatory scrutiny. A 2021 FDA warning letter cited inadequate CAPA in 41% of cases. That’s avoidable.
Use digital systems to track every CAPA. Assign owners. Set deadlines. Link it to the original defect report. At NexPCB, they found that over-reliance on statistical sampling without understanding process context led to 22% more false negatives. That’s why they added operator feedback loops-frontline workers now flag anomalies directly into the system. Human insight + machine data = stronger QC.
Why This Matters More Than Ever
Quality control isn’t a cost center. It’s a profit driver. Manufacturers spend 3.2% to 5.8% of revenue on QC. Automotive companies spend the most-5.8%-because one faulty airbag can cost millions. But the return? Higher customer retention, fewer recalls, faster regulatory approvals, and less wasted material.Global regulations are tightening. The EU’s MDR 2017/745 demands better post-market surveillance. The FDA’s new Quality Management Maturity initiative looks at culture, not just paperwork. AI-powered visual inspection is now used by 37% of Fortune 500 manufacturers. Digital twins let you simulate production before it happens. But none of this replaces the basics: clear standards, trained people, real-time monitoring, and fast fixes.
The future of QC isn’t about replacing humans with robots. It’s about giving humans better tools to make smarter decisions. As Dr. Michael Porter wrote in 2023, the most resilient systems combine Deming’s old principles with today’s tech. That’s the balance. That’s the edge.





14 Comments
lol i once saw a bottle of ibuprofen with a whole leaf inside it. no joke. the plant didnt even grow in the same country as the factory. still got sold. guess QC is just a suggestion now?
OMG YES!! I WORKED IN A PHARMA PLANT FOR 3 YEARS AND THEY WERE SO SLOPPY!! I SAW A GUY USE A DUSTY TOWEL TO CLEAN A MACHINE THAT MADE INSULIN. NO ONE SAID A WORD. THIS IS WHY PEOPLE DIE. 😭
Actually, the real issue isn't the standards-it's the culture. In places like Japan and Germany, QC isn't a department, it's a religion. Workers are trained to stop the line the second they see a deviation, even if it's a 0.01mm error. In the US, we optimize for speed and cost, and that's why we get recalls. It's not about tools-it's about mindset.
Also, ISO 9001 doesn't mandate anything-it just gives you a framework. Most companies treat it like a checkbox. That's why the FDA keeps issuing 483s. You can't audit your way to quality-you have to live it.
AI-powered visual inspection? Please. That’s just a fancy way of saying ‘we fired 20 inspectors and replaced them with a camera that can’t tell the difference between a scratch and a shadow.’ I’ve seen AOI systems miss 40% of micro-cracks because the lighting was ‘off’ that day. And now they’re telling us to trust algorithms over human eyes? 🤡
Let me be perfectly clear: Quality Control is not a cost center. It is the sacred covenant between manufacturer and consumer. When we fail to uphold measurable tolerances, we do not merely produce defective goods-we betray the fundamental trust upon which civilization is built. The CIELAB ΔE < 2.0 is not a suggestion. It is a moral imperative.
Every misaligned solder joint, every uncalibrated micrometer, every untrained inspector-these are not operational oversights. They are existential failures of character. The FDA does not issue Form 483s to punish companies. They issue them to awaken souls.
Of course they say QC is ‘critical’-because they want you to think it’s being done. But who really controls the standards? Big Pharma. Big Auto. The same corporations that lobbied to weaken the FDA’s oversight in 2018. They don’t want you to know that 70% of ‘defects’ are hidden by ‘statistical sampling’-which is just a fancy word for ‘we didn’t check most of them.’
And those ‘IoT sensors’? They’re feeding data to corporate dashboards so executives can pretend they’re ‘proactive.’ Meanwhile, the guy on the line who flagged the issue? He got written up for ‘disrupting workflow.’
Don’t believe the hype. QC is a performance. And we’re all just audience members waiting for the next recall.
They’re watching you. 😈
In Nigeria, we don’t have fancy AOI systems or digital twins. We have people. Sharp eyes. Hands that have held a thousand parts. We don’t need 1.33 Cpk-we need someone who knows when a bolt feels wrong. My uncle worked at a radio factory in Lagos for 40 years. He could tell if a capacitor was bad just by the smell of the solder.
Technology helps, yes. But never forget: the most reliable QC system is still a human who cares. In the West, we outsource empathy along with labor. That’s why we’re falling behind.
Quality isn’t measured in micrometers. It’s measured in pride.
Let me just say this: The entire QC framework is a scam designed by corporate consultants to keep you terrified of liability while they profit from your fear. Why do you think they push ‘CAPA’ so hard? Because it creates paperwork-paperwork that generates billable hours for consultants, auditors, and software vendors!
And those ‘real-time monitoring’ systems? They’re all connected to corporate servers that sell your production data to competitors. You think Siemens is sharing their Amberg plant data for free? They’re monetizing your paranoia.
And don’t get me started on ‘training.’ You think a 24-hour PowerPoint makes someone an expert? No-it makes them a compliant drone. The real QC is done by the guy who knows how to bend the rules to keep the line moving. And he’s the one who’s actually saving your life.
Wake up. This isn’t quality control. It’s control. Period.
I love how this post treats QC like some noble, quiet hero-but let’s be real: most factories have 2 people doing QC for 500 workers. And those 2 people? They’re the ones cleaning the bathrooms on weekends. The system is broken, not the people.
I worked in a plant where the QC lead had to choose between checking 100 units or finishing the paperwork to get paid. She picked paperwork. Every. Single. Day.
We need to pay QC people like engineers-not janitors.
Ugh. Another ‘best practices’ lecture from someone who’s never held a micrometer. Let me guess-you also think ‘ISO 9001’ is a religion? 🙄
I’ve seen QC departments that cost more than the product they’re inspecting. And for what? So some manager can look good in a quarterly review? Real quality isn’t in a spreadsheet. It’s in the hands of the person who built it. The rest is theater.
And AI? Please. My cousin’s drone has better image recognition than your ‘AOI system.’
Stop pretending this is science. It’s corporate cosplay.
While I appreciate the thoroughness of this breakdown, I must respectfully emphasize that the most critical yet overlooked component of any quality control system is the psychological safety of frontline personnel. When operators fear retribution for stopping the line, no amount of SPC charts or IoT sensors will mitigate systemic failure.
At Toyota, the Andon cord wasn’t just a tool-it was a symbol of trust. If a worker pulled it, the line stopped. No questions asked. No blame assigned. That cultural foundation enabled zero-defect manufacturing-not algorithms.
Until we prioritize human dignity over output metrics, we are merely optimizing failure.
Interesting post. I work in a medical device lab. We use all this tech-SPC, AOI, digital twins-but here’s the truth: 80% of our defects come from the same 3 suppliers. We’ve got the systems. We’ve got the data. But we still can’t fix the fact that someone in Shanghai is cutting corners because their client won’t pay for better materials.
So yes, QC is vital. But the real problem? Global supply chains that reward the lowest bid. No algorithm can fix that.
Also, I love that you mentioned CAPA. We had a defect last month where 12% of units had a label misalignment. Turned out the printer operator was blind in one eye. We retrained him. Fixed the issue. No blame. Just care.
That’s QC. Not the software. The person.
my cousin works at a factory that makes phone chargers and they just throw out the whole batch if one wire is loose. no testing. no logs. just ‘oops, bad day’ and start over. guess that’s why my charger caught fire
It is regrettable that the author has failed to adequately address the legal liability implications of non-compliance with 21 CFR Part 211. The absence of a formalized deviation management protocol constitutes a material breach of Good Manufacturing Practices. Such negligence, if exposed in litigation, would likely result in punitive damages exceeding $20 million per incident. One must not underestimate the gravity of regulatory non-conformance.
Write a comment